Exercise and Cognition in Schizophrenia: Is There a Link?
Schizophrenia is associated with cognitive impairment, and treatment of cognitive dysfunction in these patients represents an area of unmet need. What is the role of exercise?
Risk of Lifestyle Diseases for Patients with Schizophrenia
Improving physical activity levels and eating habits could help patients with schizophrenia.
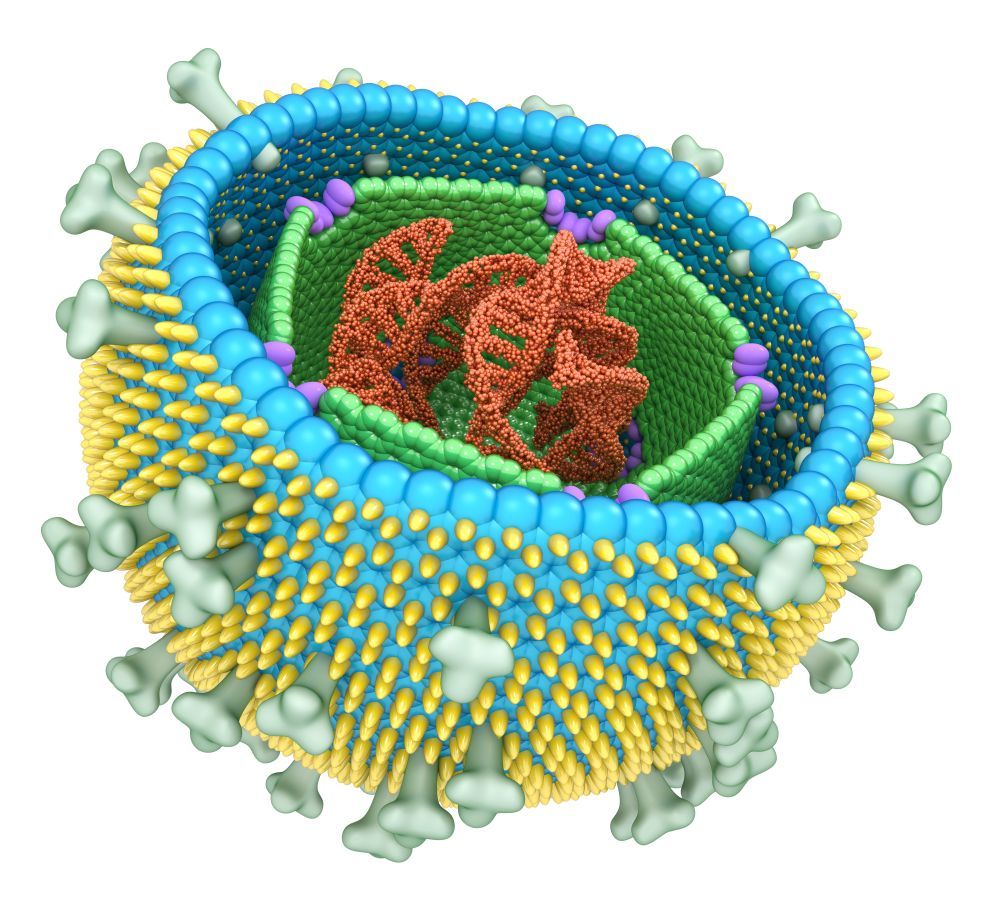
Gene Pathway Linked to Development of Schizophrenia
Researchers have discovered a gene signaling pathway associated with a higher risk for developing schizophrenia.
The Battle of the Sexes in First-Episode Psychosis
How do men versus women with schizophrenia fare clinically, functionally, and neuropsychologically over the long term?
Exposure to Epstein-Barr Virus and Cognition in Schizophrenia
Researchers explored reactivity to a panel of EBV proteins in patients with schizophrenia, thought to be associated with cognitive impairment.

An overview of schizophrenia, as well as risk factors, signs, symptoms, and treatment.
Practice guidelines for psychiatrists treating schizophrenia (undergoing copy editing, summer 2020)
Treatments for Adults with Schizophrenia Research Protocol
Patient insight and medication nonadherence data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study.
Organizational Toolkit on Medication Adherence
An overview of psychosis and schizophrenia.
Schizophrenia: Medline Plus Medical encyclopedia.
Schizophrenia genetics.
Help for military personnel and veterans: schizophrenia, treatment options, self-help tools, and resources to help overcome challenges.

What Is Schizophrenia?
Facts about schizophrenia
Includes symptom onset, age-specific information, and diagnosis/treatment.
What causes schizophrenia?
Disaster planning for people with serious mental illness.
E. Fuller Torrey, MD’s book explains the nature, causes, symptoms, treatment, and course of schizophrenia.

The SECUADO® (asenapine) transdermal system is the first and only FDA-approved patch for adults with schizophrenia. Transdermal delivery of asenapine via the patch offers your patients another treatment option.
Please see full Prescribing Information, including BOXED WARNING.
Download and fax the form to request samples or contact a rep.
Our self-guided learning module covers all things SECUADO and includes links to Important Safety Information and full Prescribing Information, including BOXED WARNING.
Help your patients access SECUADO through the Noven Care Access Network (Noven C.A.N.™).
Watch a video on the official Instructions for Use of the patch.
IMPORTANT SAFETY INFORMATION
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. SECUADO is not approved for the treatment of patients with dementia-related psychosis.
INDICATIONSECUADO® (asenapine) transdermal system is indicated for the treatment of adults with schizophrenia.
CONTRAINDICATIONS: Severe hepatic impairment (Child-Pugh C) or a history of hypersensitivity reactions to asenapine or any components of this formulation.
CEREBROVASCULAR ADVERSE EVENTS, INCLUDING STROKE: Elderly subjects with dementia had a higher incidence of stroke (including fatal stroke) and transient ischemic attack in clinical trials with antipsychotic drugs. SECUADO is not approved for the treatment of patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome (NMS): NMS, a potentially fatal symptom complex, has been reported with antipsychotics. NMS can cause hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability. If NMS is suspected, immediately discontinue SECUADO and provide intensive symptomatic treatment and monitoring.
Tardive Dyskinesia (TD): Risk of TD and the likelihood that it will become irreversible increases with the duration of treatment and the cumulative dose of antipsychotic drugs, including SECUADO. TD can develop after relatively brief treatment periods, even at low doses, and may also occur after discontinuation of treatment. Prescribe SECUADO in a manner most likely to reduce the risk of TD. If signs and symptoms of TD appear, drug discontinuation should be considered.
- Hyperglycemia and Diabetes Mellitus: Hyperglycemia, in some cases associated with ketoacidosis, hyperosmolar coma, or death, has been reported in patients treated with atypical antipsychotics. Hyperglycemia has been reported in patients treated with sublingual asenapine. Assess fasting plasma glucose before or soon after initiation of treatment, and monitor periodically during long-term treatment.
- Dyslipidemia: Atypical antipsychotics cause adverse alterations in lipids. Before or soon after initiation of antipsychotic medication, obtain a baseline fasting lipid profile and monitor periodically during treatment.
- Weight Gain: Weight gain has been observed with atypical antipsychotics, including SECUADO. Monitor weight at baseline and frequently thereafter.
Hypersensitivity Reactions: Hypersensitivity reactions, including anaphylaxis, angioedema, hypotension, tachycardia, dyspnea, wheezing, and rash have been reported in patients treated with asenapine, including SECUADO. In several cases, these reactions occurred after the first dose.
Orthostatic Hypotension, Syncope, and Other Hemodynamic Effects:Atypical antipsychotics cause orthostatic hypotension and syncope. The risk is greatest during the initial dose titration and when increasing the dose. Monitor orthostatic vital signs and patients who are vulnerable to hypotension. Use SECUADO cautiously with other drugs that can cause hypotension, bradycardia, respiratory or central nervous system depression. Consider a dose reduction if hypotension occurs.
Falls:SECUADO may cause somnolence, postural hypotension and motor or sensory instability, which may lead to falls, and consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
Leukopenia, Neutropenia, and Agranulocytosis:Leukopenia, neutropenia, and agranulocytosis (including fatal cases) have been reported with antipsychotics, including asenapine. Monitor complete blood count in patients with pre-existing low white blood cell count (WBC) or absolute neutrophil count or history of drug-induced leukopenia or neutropenia. Discontinue SECUADO at the first sign of a clinically significant decline in WBC and in severely neutropenic patients.
QT Prolongation:Sublingual asenapine was associated with increases in QTc interval from 2 to 5 msec versus placebo. There were no reports of QT prolongation exceeding 500 msec for SECUADO and placebo. The use of SECUADO should be avoided in patients with a history of cardiac arrhythmias and in circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval.
Hyperprolactinemia:SECUADO can elevate prolactin levels and the elevation can persist during chronic administration. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects.
Seizures:Use SECUADO with caution in patients with a history of seizures or with conditions that lower the seizure threshold.
Potential for Cognitive and Motor Impairment:Somnolence was reported in patients treated with SECUADO. Caution patients about operating hazardous machinery, including motor vehicles, until they are reasonably certain that SECUADO does not affect them adversely.
Body Temperature Regulation: Use SECUADO with caution in patients who will experience conditions that increase body temperature (strenuous exercise, extreme heat, dehydration and concomitant anticholinergics).
Dysphagia: SECUADO should be used cautiously in patients at risk for aspiration. Esophageal dysmotility and aspiration have been associated with antipsychotic drug use.
External Heat: Avoid direct external heat sources while wearing SECUADO.
Application Site Reactions: During wear time or immediately after removal of SECUADO, local skin irritation may occur. Instruct patients to select a different patch application site each day to limit the occurrence of skin irritation.
Adverse Reactions: Commonly observed adverse reactions (incidence ≥5% and at least twice the rate of placebo) were extrapyramidal disorder, application site reaction and weight gain.
Drug Interactions: Monitor blood pressure and adjust antihypertensive drugs when taken with SECUADO. Based on clinical response, SECUADO dose reduction may be necessary when used with strong CYP1A2 inhibitors (fluvoxamine). Reduce paroxetine (CYP2D6 substrate and inhibitor) dose by half when taken with SECUADO.
Pregnancy: Studies have not been conducted with SECUADO in pregnant women. Advise patients to notify their healthcare provider of a known or suspected pregnancy. The National Pregnancy Registry for Atypical Antipsychotics monitors pregnancy outcomes in women exposed to antipsychotics, including SECUADO®, during pregnancy. For information, contact 1-866-961-2388 or http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Please see full Prescribing Information, including BOXED WARNING.
To report suspected Adverse Reactions, contact Noven at 800-455-8070 or FDA at 800-FDA-1088 or www.fda.gov/medwatch.
SECUADO is a registered trademark of Hisamitsu Pharmaceutical Co., Inc.
NOVEN C.A.N. AND ITS RELATED LOGO ARE TRADEMARKS of Noven Therapeutics, LLC.
© 2020 Noven Therapeutics, LLC. All rights reserved. For US audience only. SDO-2337-16 07/20